But compounds containing fluorine have other practical uses that might surprise you. Scientists at the US Department of Energy’s Argonne National Laboratory have discovered a fluoride electrolyte that could protect a next generation battery against performance decline.
An exciting new generation of battery types for EVs beyond lithium ion is on the horizon. Their chemistries offer twice or more energy stored in a given volume or weight compared to lithium ion. They could power cars for much longer distances and could even power long-haul trucks and aircraft one day. The main problem is that their high energy density declines rapidly with repeated charge and discharge.
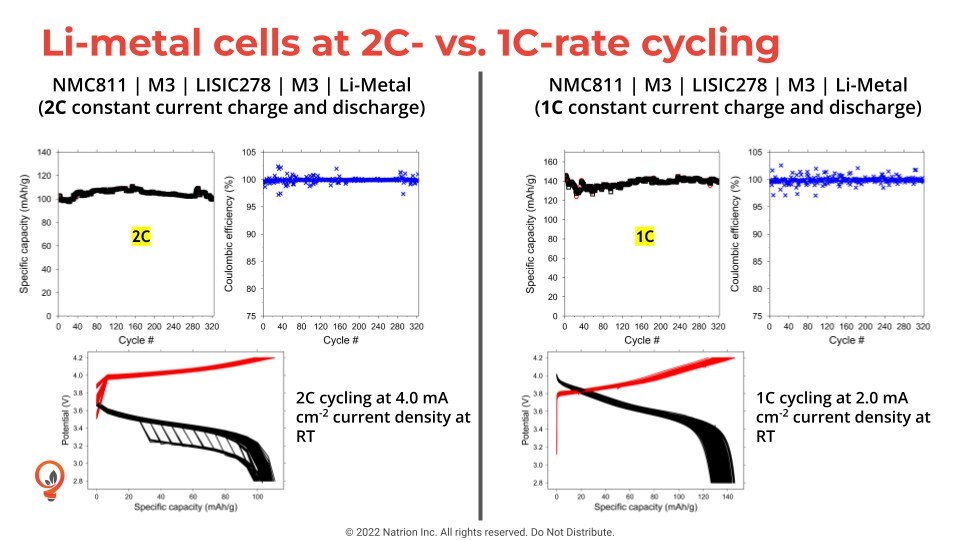
The team investigated a battery with an anode made of lithium metal in place of the graphite normally used in lithium-ion batteries. It is thus called a “lithium metal” battery. While it can deliver more than double the energy density possible with a lithium-ion battery, that outstanding performance rapidly vanishes within less than a hundred charge-discharge cycles.
The team’s solution involved changing the electrolyte, a liquid through which lithium ions move between cathode and anode to implement charge and discharge. In lithium metal batteries, the electrolyte is a liquid consisting of a lithium-containing salt dissolved in a solvent. The source of the short cycle-life problem is that the electrolyte does not form an adequate protective layer on the anode surface during the first few cycles. This layer, also called the solid-electrolyte interphase, acts like a guardian, allowing lithium ions to freely pass in and out of the anode to charge and discharge the battery, respectively.
The team discovered a new fluoride solvent that maintains a robust protective layer for hundreds of cycles. It couples a fluorinated component that is positively charged (cation) with a different fluorinated component that is negatively charged (anion).
This made all the difference in maintaining high performance for hundreds of cycles in a test cell. The team’s electrolyte offers other advantages. It could greatly increase vehicle range, cost less than current designs, be environmentally friendly because it uses much less solvent, and run more safely, since the electrolyte is nonflammable.
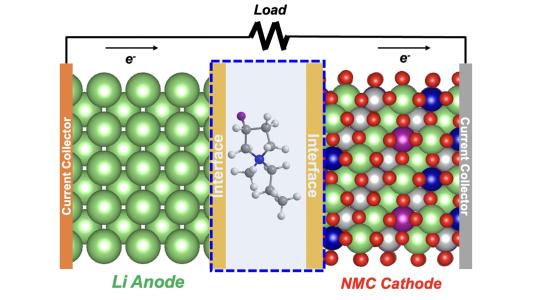
Design of lithium metal battery with electrolyte containing a fluorinated cation (atomic structure at centre). The “interface” area represents the layer with fluorine that forms on the anode surface, as well as the cathode surface. Image: Argonne National Laboratory